cesar
@C_A_ReyesPhD student @PicazoLab @USCChemistry | BS/MS @Gassensmith @UTDallasChemBio | @USMC vet
Similar User

@MerroGull26

@TSHowlett

@OTrashi

@Prashansa__K

@MeloniLab

@NiyatiA30

@shelby_phelps_

@rebeccamj916

@MadujikaA

@L_Hagge

@sra_aman

@QingYan71092994
Photoswitches that Are Compatible with Water: Stenhouse salts can serve as visible-light photoswitches in protic environments chemistryviews.org/photoswitches-…

Check out our new #SYNFORM article on the development of amino donor–acceptor Stenhouse adducts by Elias Picazo (@_Picazo) and colleagues from @USCDornsife 🔍🙌. Read more: brnw.ch/21wOF6e

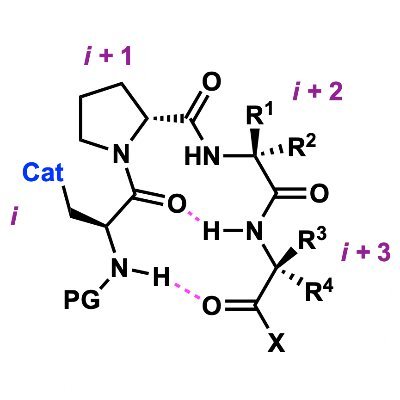
Fungibility isn't only for fiat vs #crypto (think #NFTs – how relevant with #BTC hitting ATHs)! "We recognized that the homodimer of a disulfide is the fungible disulfide itself and is, therefore, inconsequential to the transformation." We are grateful to Synlett / Synpacts for…
We're excited to share our work on direct allylic C−H sulfonylation, now out in @angew_chem Building on to our Pd(0/I/II) manifold, a diverse set of cyclic and acyclic internal alkenes are covered with post-functionalization featuring formal alkene transposition!

We are incredibly excited about this advance and look forward to learning more about the rearrangements and photoswitches. You can read more about our thoughts on the @NatureComms project through Springer Nature's "Behind the Paper" here, communities.springernature.com/posts/inducing…
!! Our most recent publication is now at Nature Communications! We developed a pyrrole rearrangement and used it to create and study a new subclass of light-responsive small molecules named amino DASAs! Congratulations to the team!! Learn more here, nature.com/articles/s4146…

Thrilled! Read Picazo group unlocks the final component on DASAs with sulfonamide protection of pyrroles! Really excited to work further on this project! Congratulations again! 🎉 @PicazoLab
!! Our most recent publication is now at Nature Communications! We developed a pyrrole rearrangement and used it to create and study a new subclass of light-responsive small molecules named amino DASAs! Congratulations to the team!! Learn more here, nature.com/articles/s4146…

Congrats team DASAs.
!! Our most recent publication is now at Nature Communications! We developed a pyrrole rearrangement and used it to create and study a new subclass of light-responsive small molecules named amino DASAs! Congratulations to the team!! Learn more here, nature.com/articles/s4146…

!! Our most recent publication is now at Nature Communications! We developed a pyrrole rearrangement and used it to create and study a new subclass of light-responsive small molecules named amino DASAs! Congratulations to the team!! Learn more here, nature.com/articles/s4146…

🔥🔥 New preprint alert. Excited to share our latest work describing the use of iron in Cross-Coupling C(sp3)–halide with soft thiol nucleophiles! Have a great read 🔥🔥🔥 #Iron #CrossCoupling #Catalysis chemrxiv.org/engage/chemrxi…

🥳 Our group was recently honored with the President’s Sustainability Initiative Award!! We are excited to further our understanding of iron catalyzed reactions! Congratulations to the group!!

Oops, we did it again! After the hugely successful carbodiimide-fueled reaction cycle, we wondered, "Can we build a similarly successful cycle but using biology's favorite reaction pair: phosphorylation-dephosphorylation?" Proud to show Simone's work: nature.com/articles/s4146…
Today Julian Götz @bode_lab @ETH_en joins to share his work from @ScienceAdvances on using high-throughput synthesis to generate data for predicting molecular properties and reaction success!! youtu.be/8F2d8B0fIpQ Thanks to our Editorial Board member Cesar Reyes for hosting!

In the fourth month of the year, we have our fourth ACIE publication out - check out those colourful fluorophores! onlinelibrary.wiley.com/doi/10.1002/an…
Neue Moleküle fluoreszieren in allen Regenbogenfarben! 🌈 Ein Team um @MaulideLab, @theo_chem & Harald Sitte von der @MedUni_Wien, hat eine Reihe neuartiger fluoreszierender Moleküle entwickelt. Warum diese in der Medizin & Pharmazie unverzichtbar sind ⤵ medienportal.univie.ac.at/media/aktuelle…
When people ask how long it’s gonna take me to finish writing my paper

I like this pic better! It shows how nitrogen's additional bonding orbital enabled a rearrangement that had been limited to furans since 1850! It also highlights how amino DASAs unlock the final static compartment in DASA photoswitches! Read more here: chemrxiv.org/engage/chemrxi…

The octupolar DASA🐙
#OnTheCover Back Cover: Robust, Ultrafast and Reversible Photoswitching in Bulk Polymers Enabled by Octupolar Molecule Design (Quan Li and co-workers) onlinelibrary.wiley.com/doi/10.1002/an… onlinelibrary.wiley.com/doi/10.1002/an…

‼️
The Piancatelli and related rearrangements have been limited to furans since 1850. We developed the first pyrrole equivalent and used it to make new light-responsive matter!! Read about the first amino DASAs in our @ChemRxiv preprint here chemrxiv.org/engage/chemrxi…

Honored because USC Chemistry featured our recently published work! 😊 USC article: dornsife.usc.edu/chemistry/2024… JACS article: pubs.acs.org/doi/10.1021/ja…

United States Trends
- 1. Thanksgiving 8.235 posts
- 2. Druski 21,9 B posts
- 3. Tyrese Martin 1.652 posts
- 4. Kevin Hart 10,8 B posts
- 5. Dylan Harper 3.711 posts
- 6. Friday Night Lights 15,9 B posts
- 7. Pat Spencer N/A
- 8. #AEWDynamite 25 B posts
- 9. #Survivor47 4.401 posts
- 10. Knicks 13,1 B posts
- 11. Zuck 10,5 B posts
- 12. Jalen Williams 1.718 posts
- 13. RJ Davis N/A
- 14. Vindman 61,1 B posts
- 15. Izzo 1.541 posts
- 16. Cruz Azul 20,2 B posts
- 17. Kuminga 2.146 posts
- 18. Ace Bailey 1.173 posts
- 19. #BillboardIsOverParty 1.119 posts
- 20. Hubert Davis N/A
Who to follow
-
 Humera Gull
Humera Gull
@MerroGull26 -
 Thomas Howlett
Thomas Howlett
@TSHowlett -
 Orikeda Trashi
Orikeda Trashi
@OTrashi -
 Prashansa Kannangara
Prashansa Kannangara
@Prashansa__K -
 Meloni Lab
Meloni Lab
@MeloniLab -
 (COF)fee o’clock
(COF)fee o’clock
@NiyatiA30 -
 Shelby Phelps
Shelby Phelps
@shelby_phelps_ -
 rebecca johnson
rebecca johnson
@rebeccamj916 -
 Madujika Apekshani
Madujika Apekshani
@MadujikaA -
 L. Hagge et al.
L. Hagge et al.
@L_Hagge -
 Aman Sra
Aman Sra
@sra_aman -
 Qing Yan
Qing Yan
@QingYan71092994
Something went wrong.
Something went wrong.